United States Vaccine Market Overview
The United States vaccine market represents a critical component of the national healthcare and public health infrastructure, playing a central role in disease prevention, outbreak control, and long-term population health management. Vaccines are widely recognized as one of the most cost-effective medical interventions, reducing morbidity, mortality, and the broader economic burden associated with infectious diseases. The market encompasses a wide range of prophylactic vaccines administered across pediatric, adult, and traveler populations through public immunization programs and private healthcare settings.
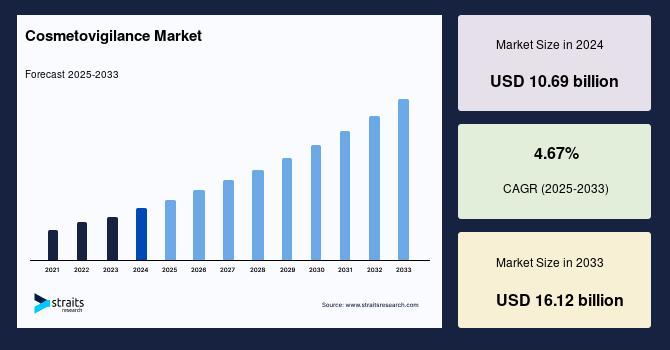
In 2025, the United States vaccine market attained a value of USD 16.27 billion. The market is projected to expand at a compound annual growth rate (CAGR) of 9.80% during the forecast period of 2026–2035, reaching an estimated value of USD 41.44 billion by 2035. Market growth is supported by strong immunization infrastructure, continuous product innovation, expanding adult and booster vaccination programs, and heightened awareness of infectious disease prevention.
Despite robust growth prospects, the market also faces structural challenges, including vaccine hesitancy, pricing pressures, supply chain complexity, and regulatory scrutiny. These factors collectively shape the competitive and operational landscape of the United States vaccine market.
Market Scope and Structure
The United States vaccine market includes preventive vaccines developed using various technologies and targeting a wide range of infectious diseases. The scope of the market covers:
Multiple vaccine technologies, including recombinant, conjugate, inactivated, live attenuated, and toxoid vaccines
Indications spanning childhood, adult, and travel-related diseases
End-use categories such as pediatric, adult, and traveler vaccines
Distribution and administration through public health programs, hospitals, clinics, and pharmacies
The market is characterized by high regulatory oversight, long development timelines, and significant investment in research, manufacturing, and post-marketing surveillance.
Key Growth Drivers
Strong National Immunization Programs
The United States benefits from well-established national immunization programs supported by federal and state governments. Organizations such as the Centers for Disease Control and Prevention (CDC) play a central role in setting immunization schedules and recommendations.
Key growth impacts include:
High baseline vaccine coverage in pediatric populations
Routine booster and catch-up vaccination programs
Stable demand supported by public funding mechanisms
These programs provide a predictable demand base for vaccine manufacturers.
Expanding Adult Vaccination Coverage
Historically, vaccination efforts were primarily focused on pediatric populations. However, adult vaccination has gained increasing attention due to aging demographics, chronic disease prevalence, and the risk of vaccine-preventable diseases in older populations.
Growth drivers include:
Increased recommendations for adult vaccines, such as influenza, pneumococcal, and hepatitis vaccines
Employer-sponsored and pharmacy-based immunization services
Greater awareness of adult immunization benefits
The expansion of adult vaccination significantly broadens the addressable market.
Rising Focus on Preventive Healthcare
Preventive healthcare has become a strategic priority within the United States healthcare system, driven by efforts to reduce long-term healthcare costs and improve population health outcomes.
Vaccines contribute to this shift by:
Reducing hospitalization rates and treatment costs
Supporting value-based healthcare models
Enhancing long-term disease control
This policy and payer-level emphasis supports sustained vaccine uptake.
Technological Advancements in Vaccine Development
Advances in vaccine technologies, including recombinant and conjugate platforms, have improved safety, efficacy, and scalability.
Key advantages include:
Enhanced immune response and longer-lasting protection
Reduced adverse event profiles compared to older technologies
Improved suitability for immunocompromised populations
Technological innovation supports lifecycle extension and new product development.
Increased Awareness of Infectious Disease Risks
Public awareness of infectious diseases and outbreak preparedness has increased over time, influencing vaccination behavior.
Market implications include:
Higher seasonal influenza vaccination rates
Increased demand for travel-related vaccines
Greater acceptance of booster immunizations
Awareness-driven demand contributes to market stability.
Market Restraints and Challenges
Vaccine Hesitancy and Misinformation
Vaccine hesitancy remains one of the most significant challenges in the United States vaccine market. Concerns related to safety, efficacy, and misinformation can negatively impact vaccination rates.
Key challenges include:
Regional and demographic variability in vaccine acceptance
Delays or refusal of routine immunizations
Increased burden on public health education efforts
Vaccine hesitancy creates uneven demand across indications and regions.
Pricing and Reimbursement Pressures
Vaccines are subject to pricing scrutiny from public payers, private insurers, and government procurement programs.
Market impacts include:
Pressure to justify pricing through clinical and economic value
Limited pricing flexibility in publicly funded programs
Margin constraints for manufacturers
Pricing dynamics influence product strategy and portfolio prioritization.
High Development and Manufacturing Costs
Vaccine development requires substantial investment in research, clinical trials, and manufacturing infrastructure.
Challenges include:
Long development timelines and regulatory uncertainty
High capital expenditure for specialized manufacturing facilities
Complex quality control and cold-chain requirements
These factors raise barriers to entry and limit the number of active players.
Supply Chain and Distribution Complexity
Vaccines often require temperature-controlled storage and distribution, adding logistical complexity.
Key risks include:
Cold-chain disruptions
Inventory management challenges during seasonal demand peaks
Dependency on centralized manufacturing facilities
Supply chain resilience is critical to market performance.
Market Trends
Shift Toward Recombinant and Conjugate Vaccines
Recombinant and conjugate vaccines are increasingly favored due to their improved immunogenicity and safety profiles.
Key trends include:
Replacement of older vaccine formulations
Expanded use in pediatric and adult populations
Support from updated immunization guidelines
This shift influences R&D priorities and manufacturing investments.
Growth of Pharmacy-Based Vaccination Services
Pharmacies have become important vaccination sites in the United States, particularly for adult and seasonal vaccines.
Market implications include:
Improved access and convenience
Higher vaccination rates among working-age adults
Expanded distribution channels for manufacturers
Retail-based immunization supports market expansion.
Emphasis on Combination Vaccines
Combination vaccines reduce the number of injections required, improving compliance and coverage.
Key benefits include:
Simplified immunization schedules
Reduced healthcare visits
Improved patient adherence
Combination products contribute to operational efficiency within immunization programs.
Increased Use of Real-World Evidence
Manufacturers and regulators increasingly rely on real-world evidence to assess vaccine effectiveness and safety post-approval.
This trend supports:
Continuous safety monitoring
Improved public confidence
Data-driven policy decisions
Market Segmentation Analysis
Market Breakup by Technology
Recombinant and Conjugate Vaccines
This segment represents a significant and growing share of the market due to superior immunological performance and safety.
Key drivers include:
Broad applicability across age groups
Strong support from immunization guidelines
Ongoing product innovation
Inactivated Vaccines
Inactivated vaccines remain widely used for diseases such as influenza and polio.
Strengths include:
Established safety profiles
Suitability for immunocompromised patients
Live Attenuated Vaccines
Live attenuated vaccines offer strong immune responses but are used selectively due to contraindications in certain populations.
Toxoid Vaccines
Toxoid vaccines continue to play an important role in preventing toxin-mediated diseases such as tetanus and diphtheria.
Others
Includes emerging and specialized vaccine technologies.
Market Breakup by Indication
Pneumococcal Disease
Pneumococcal vaccines are widely used in pediatric and elderly populations, supporting consistent demand.
Influenza
Influenza vaccines represent one of the largest segments due to annual administration and seasonal outbreaks.
Human Papilloma Virus (HPV)
HPV vaccination is supported by cancer prevention initiatives and expanding age-based recommendations.
Meningococcal Disease
Meningococcal vaccines are commonly used in adolescents and high-risk groups.
Rotavirus
Primarily administered in pediatric populations as part of routine immunization schedules.
Varicella, Measles, Mumps, and Rubella
Combination vaccines in this category support high pediatric coverage rates.
Diphtheria, Pertussis, and Tetanus (DPT)
DPT vaccines remain essential components of childhood and booster immunization programs.
Polio
Polio vaccines continue to be administered as part of routine prevention efforts.
Hepatitis
Hepatitis vaccines serve both pediatric and adult populations, including high-risk groups.
Others
Includes vaccines for less common or emerging infectious diseases.
Market Breakup by End Use
Pediatric Vaccines
Pediatric vaccines account for a substantial share of the market due to mandatory immunization schedules and high coverage rates.
Adult Vaccines
Adult vaccines represent a growing segment, driven by aging populations and expanded recommendations.
Travelers Vaccines
Travel-related vaccines serve individuals traveling to regions with specific disease risks, contributing incremental demand.
Regional Analysis
North America
North America, led by the United States, represents the largest regional market due to advanced healthcare infrastructure, strong regulatory frameworks, and high immunization awareness.
Europe
Europe contributes significantly through established immunization programs and public healthcare systems.
Asia Pacific
Asia Pacific represents a growing opportunity due to population size and expanding vaccination initiatives.
Latin America
Market growth in Latin America is supported by public health programs and international partnerships.
Middle East and Africa
These regions represent emerging markets with improving immunization coverage and infrastructure.
Competitive Landscape
The United States vaccine market is characterized by a concentrated competitive structure, with a limited number of multinational pharmaceutical companies dominating the landscape.
Companies Covered
GlaxoSmithKline plc
Merck & Co., Inc.
Novartis AG
Pfizer Inc.
Sanofi
AstraZeneca plc
Johnson & Johnson Services, Inc.
Others
Competition is driven by portfolio breadth, manufacturing capacity, regulatory compliance, and long-term government relationships.
Future Outlook
The United States vaccine market is expected to maintain strong growth momentum through 2035, supported by expanding adult immunization, technological innovation, and continued emphasis on preventive healthcare. While challenges such as vaccine hesitancy, pricing pressures, and supply chain complexity persist, the market benefits from strong institutional support and a well-established regulatory environment.
Future growth will be shaped by:
Continued innovation in vaccine technologies
Expansion of adult and booster vaccination programs
Strengthening of distribution and cold-chain infrastructure
Data-driven public health policy and surveillance
Overall, the United States vaccine market is positioned for sustained, long-term growth, underpinned by its central role in public health and disease prevention.
Find More Reports
Osteoarthritis Therapeutics Market
About Us:
Expert Market Research is a leading market research firm delivering data-driven insights to the pharmaceutical, biotechnology, and medical device industries. Our comprehensive research solutions include market research reports, providing in-depth analysis of industry trends and competitive landscapes; drug pipeline reports, tracking drug development progress, clinical trials, and regulatory approvals; epidemiology reports, offering detailed disease prevalence and patient population studies; and patent reports, assessing intellectual property landscapes and innovation trends, among others.
Leveraging proprietary data, advanced analytics, and expert methodologies, we help businesses navigate complex markets, optimize strategies, and drive innovation. We empower clients with actionable intelligence, enabling them to make informed decisions and stay ahead in the rapidly evolving healthcare sector.
Media Contact:
Company Name: Claight Corporation
Contact Person: Roshan Kumar, Digital Marketing
Email: sales@expertmarketresearch.com
Toll-Free Number: US +1-415-325-5166 | UK +44-702-402-5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com